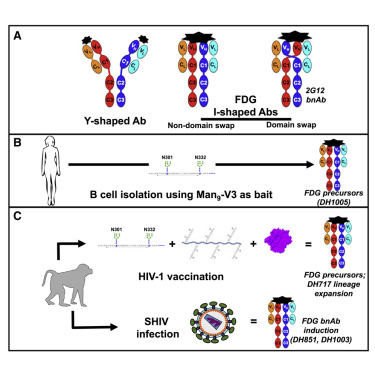
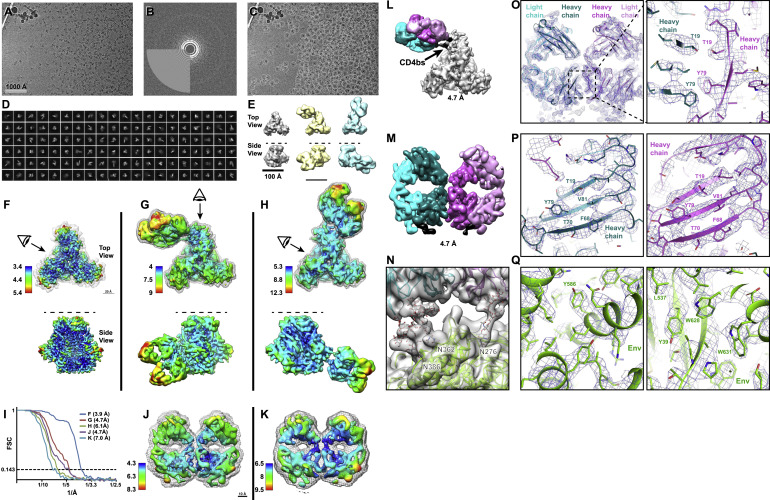
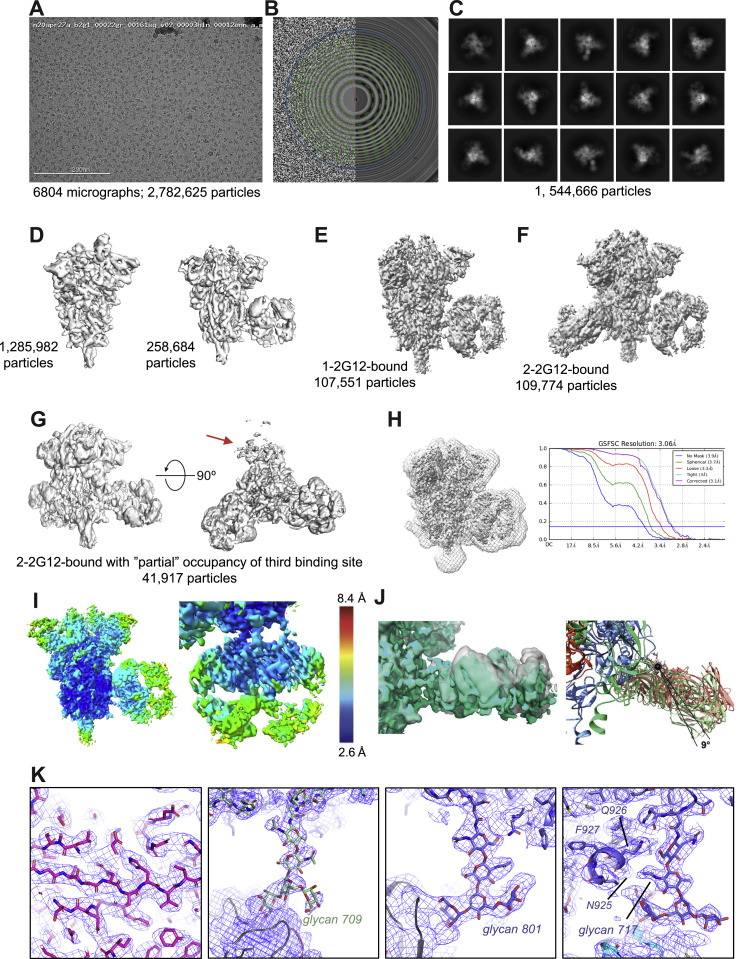
Fab-dimerized glycan-reactive antibodies are a structural category of natural antibodies
Natural antibodies (Abs) can target host glycans on the surface of pathogens. We studied the evolution of glycan-reactive B cells of rhesus macaques and humans using glycosylated HIV-1 envelope (Env) as a model antigen. We describe HIV-1 Env Fab-dimerized glycan (FDG)-reactive neutralizing Abs in HIV-1 vaccinated and simian-HIV (SHIV)-infected macaques, and HIV-1 naïve humans. FDG Abs also recognized cell-surface glycans on diverse pathogens, including yeast and SARS-CoV-2 spike. FDG precursors were expanded by glycan-bearing immunogens in macaques and were abundant in HIV-1-naïve humans. Moreover, FDG precursors were predominantly IgM and mutated, suggesting that they originated from a pool of antigen-experienced IgM+ or marginal zone-like B cells.
| Abstract | Natural antibodies (Abs) can target host glycans on the surface of pathogens. We studied the evolution of glycan-reactive B cells of rhesus macaques and humans using glycosylated HIV-1 envelope (Env) as a model antigen. 2G12 is a broadly neutralizing Ab (bnAb) that targets a conserved glycan patch on Env of geographically diverse HIV-1 strains using a unique heavy-chain (VH) domain-swapped architecture that results in fragment antigen-binding (Fab) dimerization. Here, we describe HIV-1 Env Fab-dimerized glycan (FDG)-reactive bnAbs without VH-swapped domains from simian-human immunodeficiency virus (SHIV)-infected macaques. FDG Abs also recognized cell-surface glycans on diverse pathogens, including yeast and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike. FDG precursors were expanded by glycan-bearing immunogens in macaques and were abundant in HIV-1-naive humans. Moreover, FDG precursors were predominately mutated IgM+IgD+CD27+, thus suggesting that they originated from a pool of antigen-experienced IgM+ or marginal zone B cells. |
|---|