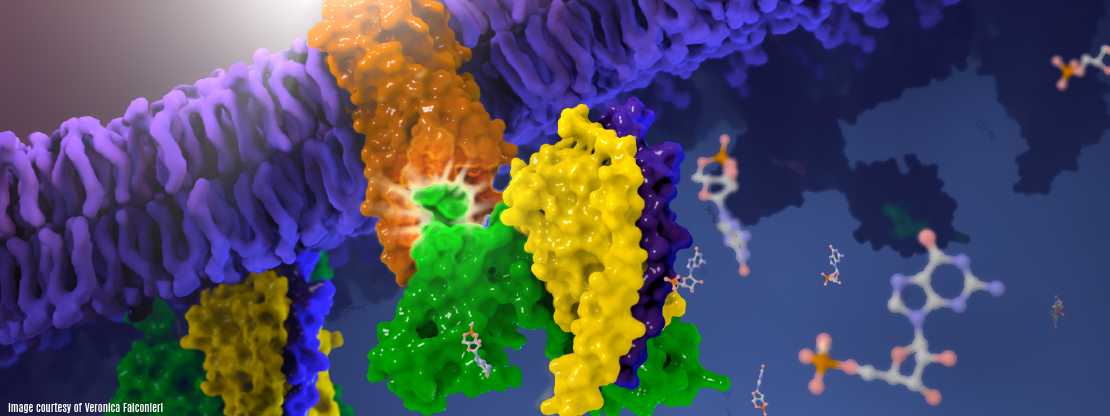
Cryo-EM structure of human rhodopsin bound to an inhibitory G protein
For the first time, scientists have visualized the interaction between two critical components of the body’s vast cellular communication network, a discovery that could lead to more effective medications with fewer side effects for conditions ranging from migraine to cancer. The near-atomic resolution images obtained, show a G-protein coupled receptor (GPCR) called rhodopsin bound to an inhibitory G protein, and provides a blueprint for designing more precise, selective drugs while also solving a longstanding problem in the field.
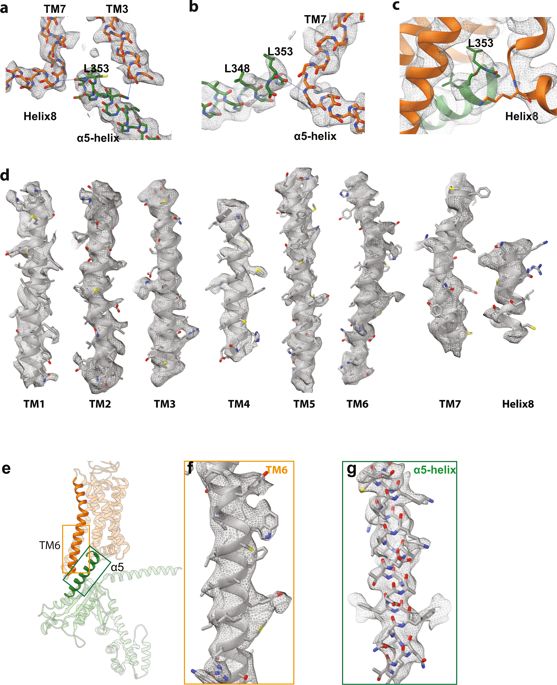
| Abstract | G-protein-coupled receptors comprise the largest family of mammalian transmembrane receptors. They mediate numerous cellular pathways by coupling with downstream signalling transducers, including the hetrotrimeric G proteins Gs (stimulatory) and Gi (inhibitory) and several arrestin proteins. The structural mechanisms that define how G-protein-coupled receptors selectively couple to a specific type of G protein or arrestin remain unknown. Here, using cryo-electron microscopy, we show that the major interactions between activated rhodopsin and Gi are mediated by the C-terminal helix of the Gi α-subunit, which is wedged into the cytoplasmic cavity of the transmembrane helix bundle and directly contacts the amino terminus of helix 8 of rhodopsin. Structural comparisons of inactive, Gi-bound and arrestin-bound forms of rhodopsin with inactive and Gs-bound forms of the β2-adrenergic receptor provide a foundation to understand the unique structural signatures that are associated with the recognition of Gs, Gi and arrestin by activated G-protein-coupled receptors. |
|---|